Surgical Devices Retrieval Endo Bags 200ml Volume for Cholecystectomy
Overview Surgical devices Retrieval endo bags 200ml volume for Cholecystectomy--top 3 China manufacturer with 17 years m
Send your inquiryDESCRIPTION
OverviewBasic Info.
| Model NO. | HSD-100/C |
| Customized | Yes |
| Diameter | 5mm |
| Transport Package | Blister Package |
| Specification | 1000ml volume |
| Trademark | TK |
| Origin | China |
| HS Code | 901890 |
| Production Capacity | 300000 Pieces/Year |
Product Description
Surgical devices Retrieval endo bags 200ml volume for Cholecystectomy--top 3 China manufacturer with 17 years manufacturing experience of medical devicesGTK Strong Quality System
GTK Medical, established on March 3, 2004, is a hi-tech medical device manufacturer which focuses on the R&D and manufacturing surgical devices like trocars, retrieval bags for more than 17 years.
GTK Core StrengthGTK Medical passed GMP of NMPA(CHINA FDA) AND US FDA factory registration (No. 3008191256) and ISO13485:2016 medical devices quality management system.

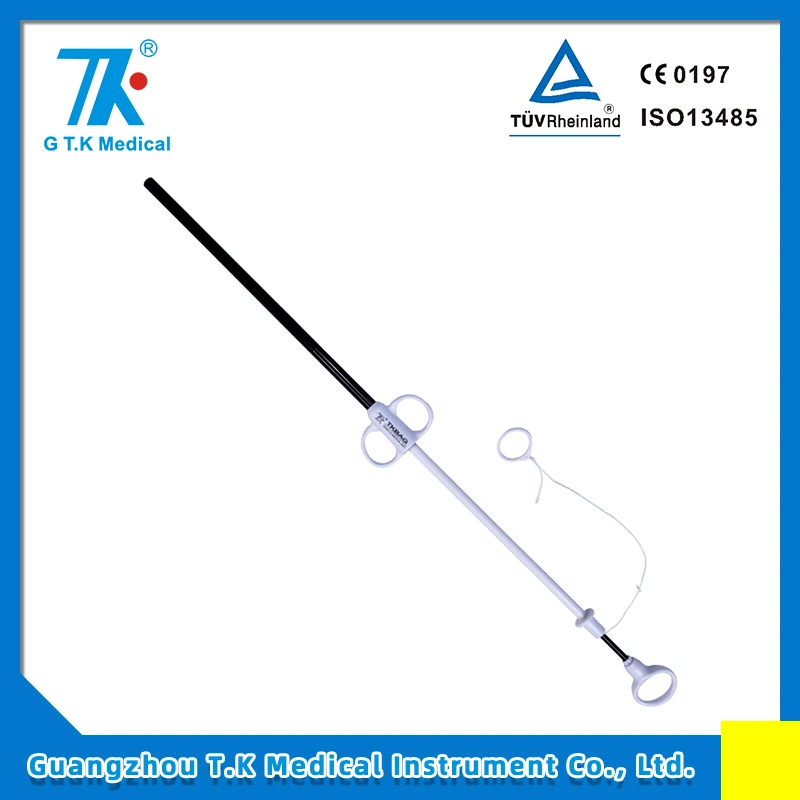

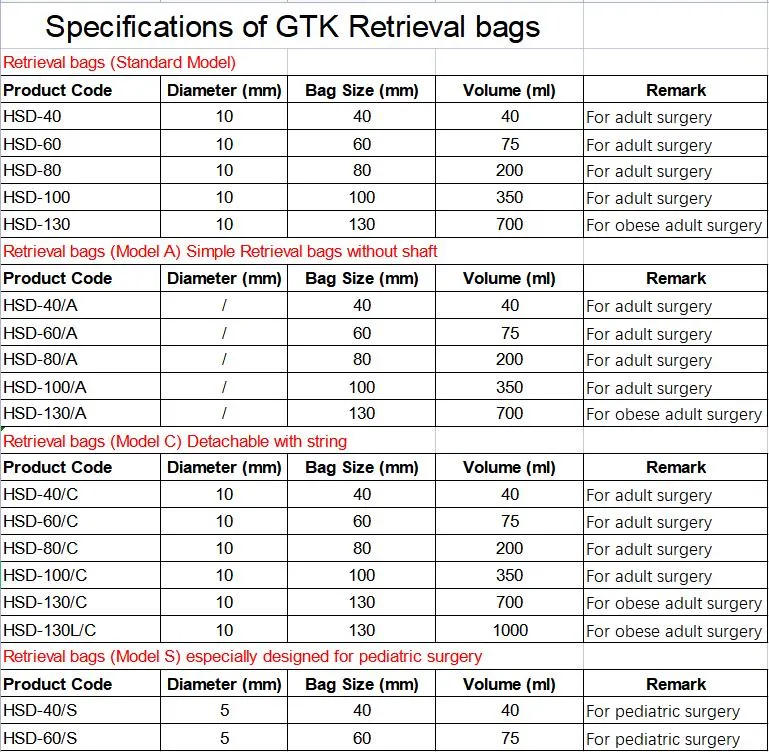

Q&A on GTK Retrieval Bags;1. Q: What is the core strength of GTK Medical? A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products.2. Q: What makes GTK Retrieval Bags stand out? A: Excellent retrieving performance plus excellent soft medical bag to ensure safety and efficacy.3. Q: Do you provide free samples of GTK Retrieval Bags for clinical trial use? A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation.4. Q: Do you have exported experiences to large medical devices company/companies? A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe.5. Q: Do you accept factory audit prior to formal partnership? A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021.
Send me an inquiry, let's embrace a win-win business relation~I will provide you with best price~We will never let you down~Contact me right now~
Related Products
-
![High Quality Trauma Implant Orthopedic Plate Medical Supply, Power Tools, Orthopedic Instruments]()
High Quality Trauma Implant Orthopedic Plate Medical Supply, Power Tools, Orthopedic Instruments
-
![Endoscope Instrument Human Staples Disposable Endoscopic Linear Stapler for Laparoscope]()
Endoscope Instrument Human Staples Disposable Endoscopic Linear Stapler for Laparoscope
-
![Hot Sales Laparoscopic Hemo Lok Clip Applier, Reusable Polymer Plastic Vascular Endo Clip Applier China Manufacture]()
Hot Sales Laparoscopic Hemo Lok Clip Applier, Reusable Polymer Plastic Vascular Endo Clip Applier China Manufacture
-
![Real Surgical Instruments Disposable Laparoscopic Specimen Endo Retrieval Bag]()
Real Surgical Instruments Disposable Laparoscopic Specimen Endo Retrieval Bag